
The important part is just to be aware of the different energy levels of the subshells when they're unoccupied versus occupied.
However I find it easier to write them as 4s3d since it follows the order of the pyramid Sal has in the upper left, so I use that format instead. So technically the electron configurations for elements like scandium or titanium should be written like 3d4s since they are filling those subshells causing the new energy difference as compared to the subshells being unoccupied. Now when filled the 3d subshell would be written first since it has lower energy giving us 3d4s. However once filled, the 3d subshell actually becomes less energetic and has lower energy than the filled 4s orbital. So when the 4s and 3d subshells are unoccupied by electrons the 4s is lower than the 3d subshell so we would fill the 4s first and write it first giving us 4s3d. The problem is when we start filling them for transition metals. So the two ways of writing the electron configurations are having a disagreement about which subshell is lower in energy. So this would mean writing the lower energy subshells further to the left and the higher ones toward the right.
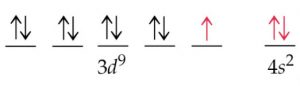
When writing electron configurations the aufbau principal says that electron subshells with lower energy should be filled first. The difference arises due to the energy levels of the two subshells. Short answer, technically the more correct way would be to write 3d4s, but it is still acceptable to write 4s3d. There is some debate which way is better, either 4s3d or 3d4s, for describing the electron configurations of transition metals.


 0 kommentar(er)
0 kommentar(er)
